Diketahui Ksp AgCl = 1 x 10⁻¹⁰. Kelarutan AgCl dal...
The equilibrium constant for a dissolution reaction, called the solubility product ( Ksp ), is a measure of the solubility of a compound. Whereas solubility is usually expressed in terms of mass of solute per 100 mL of solvent, Ksp is defined in terms of the molar concentrations of the component ions. In contrast, the ion product ( Q) describes.

Soal Tentukan rumus Ksp dari CuCl!
Let's focus on one step in Practice Problem 4 . We started with the solubility product expression for Ag 2 S. Ksp = [Ag +] 2 [S 2-] We then substituted the relationship between the concentrations of these ions and the solubility of the salt into this equation. [2 Cs] 2 [ Cs ] = 6.3 x 10 -50.
36. The ksp of AgCrO4,AgCl,AgBr and AgI are respectively, 1.1*10** 12,1.8*10** 10,5*10** 13,8.3
Using the value of Ksp for Ag2S, Ka1 and Ka2 for H2S and Kf = 1.1 x 10^5 for AgCl2-, calculate the equilibrium constant for the following reaction: Ag2S (s) + 4Cl- <<->> 2AgCl2- (aq) + H2S (aq) There are 2 steps to solve this one.

Tuliskan Persamaan Ion dan RumusRumus Ksp nya, jika zatzat berikut dilarutkan ke dalam air
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl.This white crystalline solid is well known for its low solubility in water and its sensitivity to light.Upon illumination or heating, silver chloride converts to silver (and chlorine), which is signaled by grey to black or purplish coloration in some samples.

Rumus Kelarutan S My XXX Hot Girl
K sp = [Ce 4+] [IO 3 ¯] 4. We know the following: There is a 1:1 molar ratio between the molar solubility and the cerium (IV) ion concentration. These is a 1:4 molar ratio between the molar solubility and the iodate ion concentration. Therefore: K sp = (1.80 x 10¯ 4) (7.20 x 10¯ 4) 4. K sp = 4.84 x 10¯ 17.

Rumus hasil kali kelarutan (KSP) Ag2CrO4 dinyatakan sebag...
The Ksp for AgCl is 1.6 x 10-10 at 25°C, a very insoluble compound. Sample calculations: Calculate the concentration of silver ion at room temperature (25°C) in a saturated solution of silver chloride. Click here for answer. Calculate the concentration of fluoride ion for BaF 2 if its Ksp is 2.4 x 10-5 at 25°C.

Rumus hasil kali kelarutan (KSP) Ag2CrO4 dinyatakan sebag...
Diperoleh rumus Ksp AgCl sebagai berikut: Ksp = [Ag^+][Cl^-] Ksp = s.s Ksp = s^2 Dengan demikian, rumus Ksp AgCl adalah s^2 dengan s = kelarutan AgCl. Beri Rating · 0.0 (0) Balas. Iklan. Iklan. Yah, akses pembahasan gratismu habis. Dapatkan akses pembahasan sepuasnya tanpa batas.
the galvanic cell Ag AgCl(s), KCI(0.2M) KBr(0.001IM), AgBr(s) Ag The anode is[ Ksp (AgCI) 2.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Calculate the value of the equilibrium constant for the following reaction: AgCl (s) + Cl- (aq) -------> AgCl2- (aq) Kc= ? The solubility product constant, Ksp, for AgCl is 1.77× 10-10 and the formation constant, Kf, for AgCl2- is 1.8.

Calculate The Molar Solubility Of Agcl At C Ksp X X M Hot Sex Picture
Ksp = (Ag +) 2 (CrO 4 2-) 4.10-12 = (2s) 2 (s) 4.10-12 = 4s 3. 10-12 = s 3. s = 10-4 ( CrO 4 2-) s = 10-4. D. Pengaruh Ion Senama Terhadap Kelarutan. Ion senama adalah ion yang sejenis dengan ion-ion yang ada dalam sistem keseimbangan. Untuk menerapkan rumus ion senama harus menggunakan asas. Asas yang dimaksud adalah Asas Le Chatelier. Asas Le.
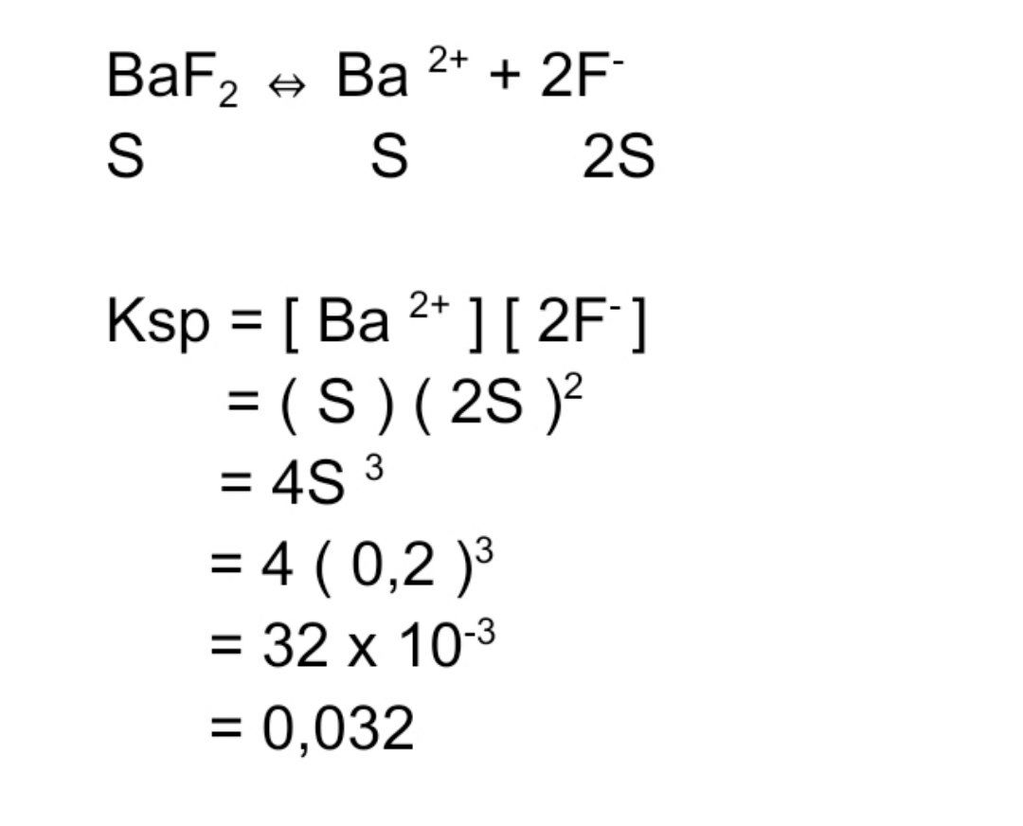
Tentukan besarnya Ksp senyawa berikut. 350 gram Ba...
It is meaningless to compare the solubilities of two salts having different formulas on the basis of their Ks values. Example 17.2.2 17.2. 2. The solubility of CaF 2 (molar mass 78.1) at 18°C is reported to be 1.6 mg per 100 mL of water. Calculate the value of Ks under these conditions.

Diketahui Ksp AgCl=1 10^10. Kelarutan AgCl dalam larutan...
M x A y (s) → x M y+ + y A x-. Karena zat pada tidak mempunyai molaritas, maka tetapan kesetimbangan reaksi diatas hanya melibatkan ion-ionnya saja, dan tetapan kesetimbangannya disebut tetapan hasil kali kelarutan (Ksp).. Ksp = [M y+] x [A x-] y. Hubungan Kelarutan dengan Ksp. Secara umum, hubungan antara kelarutan (s) dengan tetapan hasil kali kelarutan (Ksp) untuk larutan elektrolit A x B.

Rumus Tetapan Hasil Kali Kelarutan Ksp YouTube
Kalau Ksp = Qsp, artinya larutan berada di titik jenuh. Ini adalah batas sebelum munculnya pengendapan. Kalau Ksp < Qsp, ada zat padat yang mengendap. Ilustrasi campuran zat yang larut, berada pada titik jenuh, dan mengendap (Arsip Zenius) Baca Juga: Pengertian dan Rumus Larutan Penyangga - Materi Kimia Kelas 11.

Tuliskan rumus Ks p untuk senyawasenyawa berikut.a. A...
Question: Calculate the value of the equilibrium constant, Kc, for the reaction AgCl (s) + Cl- (aq) AgCl2 (aq) Kc = ? The solubility product constant, Ksp, for AgCl is 1.77 x 10-10 and the overall formation constant, Kp (B2), for AgCl, is 1.8 x 105. K. Show transcribed image text. Here's the best way to solve it.

calculate the molar solubility of AgCl if its Ksp is 1 8 x 10^8 (i) in aqueous solution (ii
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of 2AgCl = 2Ag + Cl2, the equation is balanced.

Diketahui harga hasil kali kelarutan (Ksp) dari senyawas...
Tetapan Hasil Kali Kelarutan (Ksp) - RumusKimia.net kali ini akan berbagi materi kimia tentang Rumus Tetapan Hasil Kali Kelarutan dan Contoh Soal Penyelesaian. Dalam suatu larutan jenuh dari suatu elektrolit yang sukar larut, terdapat kesetimbangan antara zat padat yang tidak larut dan ion-ion zat itu yang larut. MxAy (s) ⇄ x My+(aq) + y Ax.

Cara Mentukan Rumus Ksp Kimia Bit CDN
7.9 x 10 -15. Fe (OH) 3. 6.3 x 10 -38. Pb (OH) 2. 2.8 x 10 -16. Mg (OH) 2. 1.5 x 10 -11. Mn (OH) 2. 4.6 x 10 -14.