
Dichlorine pentoxide to form ch... Physical Chemistry
Dichlorine pentoxide (Cl. 2. O. 5. ) Molecule Lewis Structure. Dichlorine pentoxide (Cl 2 O 5) contains two chlorine and five oxygen atoms. In the lewis structure of Cl 2 O 5 molecule there are three Cl=O bonds. One chlorine atom is located as a center atom and other chlorine atom is located in a side of the molecule.

PPT Mixed Formula Practice PowerPoint Presentation, free download ID2170520
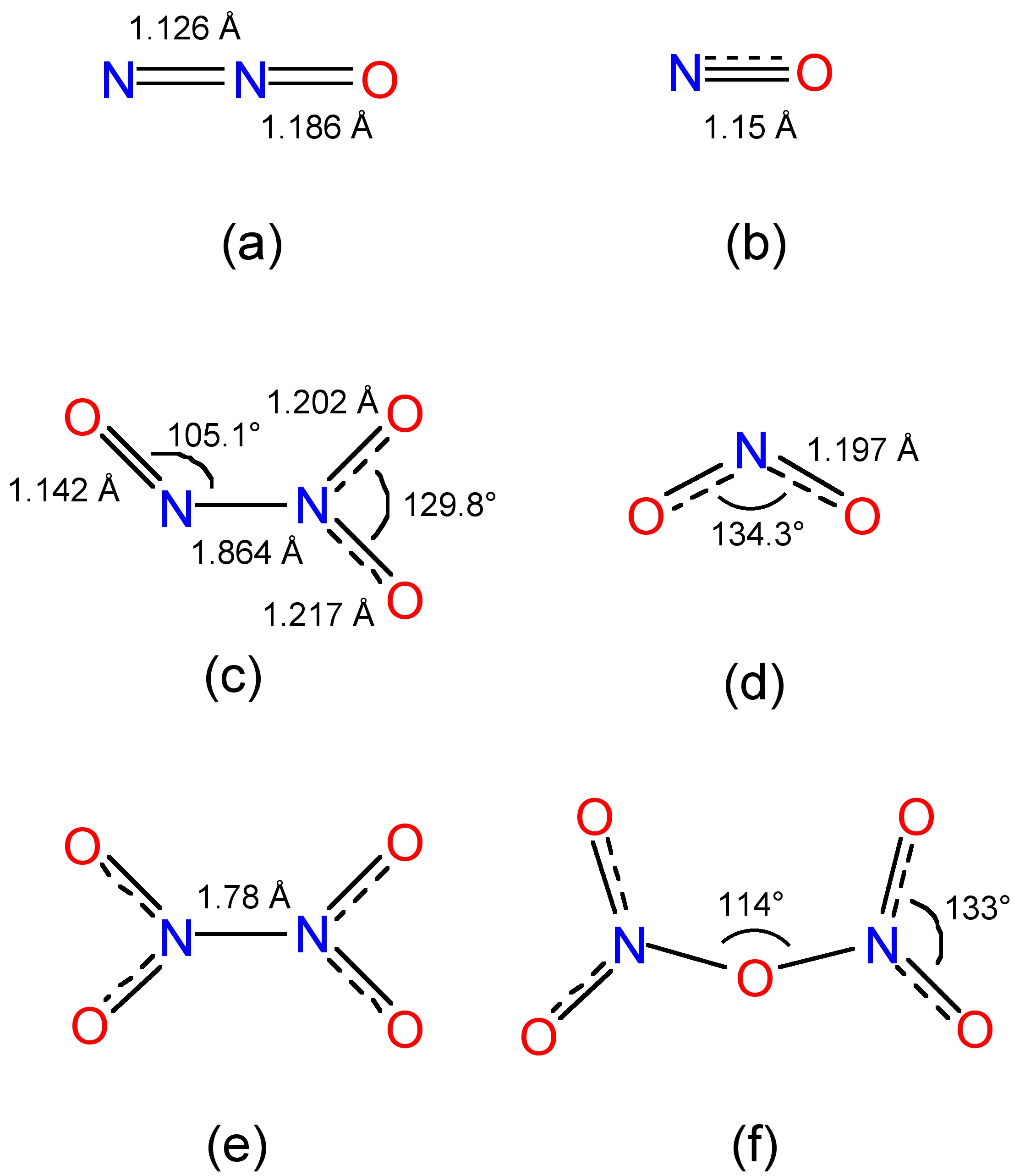
Dichlorine pentoxide is a hypothetical chlorine oxide with a chemical formula Cl2O5. The most stable configuration of dichlorine pentoxide is unknown, but theory predicts that the perchloryl/chloride peroxide structure would be the most stable among various isomers, such as the anhydride of chloric acid or the chlorous acid/perchloric acid mixed anhydride.

PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free download ID1267929
Dichlorine Pentoxide Cl2O5 Molar Mass, Molecular Weight. Cl2O5 Molar Mass Converter

Rumus Kimia Sulfur Tetrafluorida Rumus Kimia
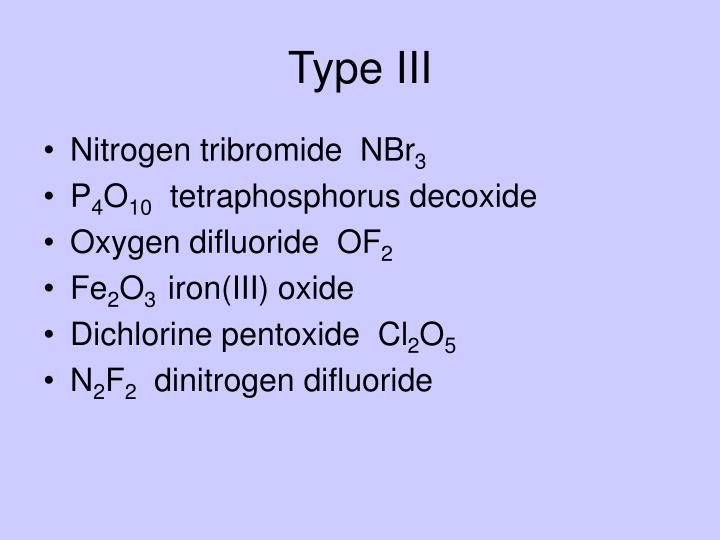
= Dichlorine pentoxide (Cl 2 O 5) Perchloric acid (HClO 4) = Dichlorine heptoxide (Cl 2 O 7) Dichlorine monoxide, Cl 2 O, is one of the "chlorine-oxide" creators of the oxy-chlorine acids. It is a brownish yellow gas at room temperature that can explode at high concentrations when heated or subjected to sparks.

Binary Molecular Compounds ppt download
Dichlorine pentoxide is a hypothetical chlorine oxide with a chemical formula Cl 2 O 5. The most stable configuration of dichlorine pentoxide is unknown, but theory predicts that the perchloryl /chloride peroxide structure would be the most stable among various isomers, [1] such as the anhydride of chloric acid or the chlorous acid / perchloric.
8.4 Oxides and Oxoacids Chemistry LibreTexts
dichlorine trioxide, Cl2O3 as hypothetical isomer O−Cl−O−Cl−O, chlorine (III) oxide. dichlorine tetroxide, also known as chlorine perchlorate, Cl2O4 or ClOClO3, chlorine (I,VII) oxide. dichlorine pentoxide, Cl2O5 or ClOOClO3, is hypothetical. dichlorine hexoxide or chloryl perchlorate, Cl2O6 or [ClO2]+[ClO4]−, chlorine (V,VII) oxide.

SOLVEDWrite the formulas of the following compounds. a. nitrogen tribromide b. xenon tetroxide
Dichlorine pentoxide is a highly reactive compound. It is a powerful oxidizing agent and can react violently with various substances, such as water, organic compounds, reducing agents, and even some metals. This compound is highly reactive and has the potential to be a powerful oxidizing agent. When it comes into contact with water, it reacts.
Chlorine Trifluoride Dichlorine Monoxide Chemistry Chloride, PNG, 1100x891px, Chlorine
Dichlorine pentoxide is a hypothetical chlorine oxide with a chemical formula Cl 2 O 5. The most stable configuration of dichlorine pentoxide is unknown, but theory predicts that the perchloryl /chloride peroxide structure would be the most stable among various isomers, [1] such as the anhydride of chloric acid or the chlorous acid / perchloric.

WANIBESAK Antimon Pentaoksida Manfaat, Sifat Fisika, Pembuatan, Reaksi Kimia
Dichlorine heptoxide is the chemical compound with the formula Cl 2 O 7.This chlorine oxide is the anhydride of perchloric acid.It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:. 2 HClO 4 + P 4 O 10 → Cl 2 O 7 + H 2 P 4 O 11. The chlorine(VII) oxide can be distilled off from the mixture.
SOLVED Write formulas for each of the following compounds. Click in the palette 4 (3) lead(I
Composition of Dichlorine Pentoxide - O 5 Cl 2. Element Symbol Atomic Mass # of Atoms Mass Percent; Oxygen: O: 79.997 g/mol: 5: 53.0122%: Chlorine: Cl: 70.906 g/mol: 2: 46.9878%: Element - Mass Percent Oxygen 79.997g Oxygen 79.997g Chlorine 70.906g Chlorine 70.906g. 🛠️ Calculate Molar Mass. Instructions.
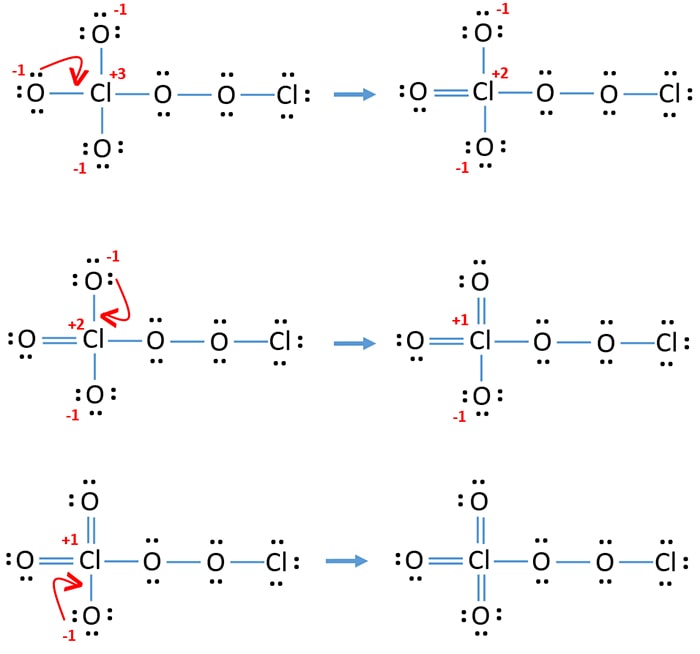
Dichlorine pentoxide (Cl2O5) Molecule Lewis Structure
Dichlorine Pentoxide - Cl 2 O 5. Cl2O5 Molar Mass Cl2O5 Oxidation Number. Reaction Expressions. Equilibrium Constant & Reaction Quotient. K c or Q = ( [Cl 2 O 5] 2) / ( [Cl 2] 2 [O 2] 5) (assuming all reactants and products are aqueous. substitutue 1 for any solids/liquids, and Psubstance for gases.)

Diklorin Pentaoksida Mempunyai Rumus Kimia UNDIGI
To find the correct oxidation state of Cl in Cl2O5 (Dichlorine pentoxide), and each element in the molecule, we use a few rules and some simple math.First, s.
Dichlorine Monoxide Arsenic Pentoxide Hypochlorous Acid PNG, Clipart, Anhidruro, Arsenic
Senyawa Diklorin Pentaoksida termasuk senyawa yang sangat berbahaya jika tidak ditangani dengan benar. Senyawa ini dapat menimbulkan iritasi pada kulit, mata, dan saluran pernapasan. Jika terhirup dalam jumlah banyak, senyawa ini dapat menyebabkan kerusakan paru-paru dan bahkan kematian. Oleh karena itu, penting untuk selalu menggunakan alat.

30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende
Dichlorine pentoxide is a hypothetical chlorine oxide with a chemical formula Cl 2 O 5. The most stable configuration of dichlorine pentoxide is unknown, but theory predicts that the perchloryl /chloride peroxide structure would be the most stable among various isomers, [1] such as the anhydride of chloric acid or the chlorous acid / perchloric.
PPT Nomenclature for Binary Compounds PowerPoint Presentation ID1155529
Dichlorine Pentoxide Formula. Dichlorine Pentoxide is a chemical compound which can be represented using the chemical symbol Cl 2 O 5.It is an organic compound which has a molar mass of 150.903 g/mol.
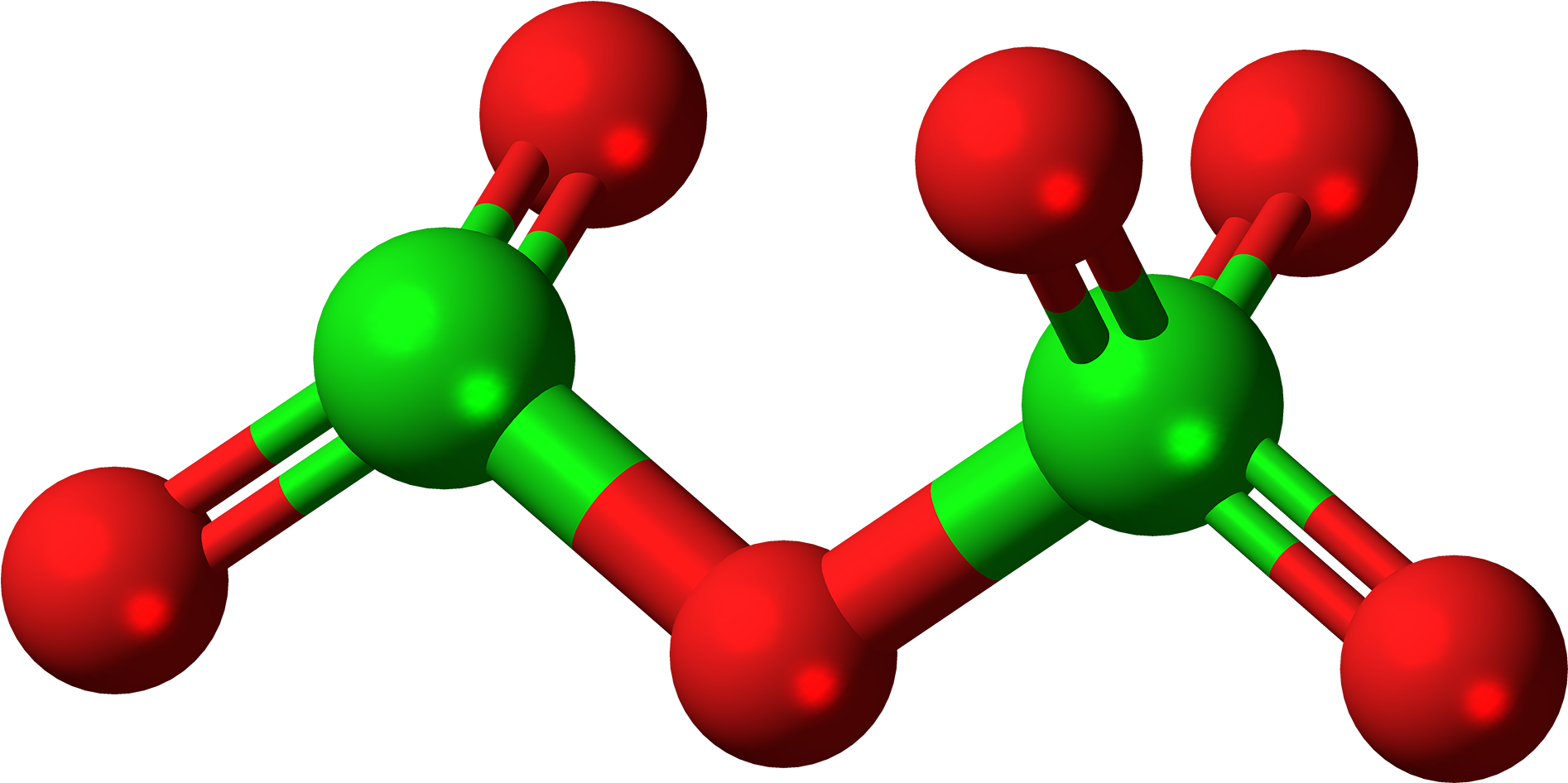
Download HD Dichlorinehexoxide Molecule Ball Dichlorine Pentoxide Transparent PNG Image
HX2O +ClX2OX5 2HClOX3 H X 2 O + C l X 2 O X 5 2 H C l O X 3. The statement in the first article and the simple fact that there is such a dearth of information about this compound suggest that it really only exists as a transient, unstable, oxidizing acid that apparently has not been isolated as a stable compound.