
What is Oxidizing Gas?
12.7: Oxidizing Agents. The laboratory oxidation of an alcohol to form an aldehyde or ketone is mechanistically different from the biochemical oxidations with NAD (P) + that we saw earlier in this chapter. The general picture of laboratory oxidations is illustrated below. Essentially what happens is that the hydroxide hydrogen of the alcohol is.

Identifying Oxidizing and Reducing Agents from the Reactions, Chemistry Lecture Sabaq.pk YouTube
Apa itu Oksidator? Ini adalah simbol bahaya untuk oksidator. Oksidator, juga dikenal sebagai oksidan atau zat pengoksidasi, adalah reaktan yang menghilangkan elektron dari reaktan lain selama reaksi redoks . Ini juga dapat dianggap sebagai spesies kimia yang mentransfer atom elektronegatif ke substrat. Asal kata berasal dari transfer oksigen.

Warning Oxidizing Substance Sign health and safety signage
Cu2+(aq) is the oxidizing agent because it gains two electrons, decreasing from an oxidation state of +2 in Cu2+(aq) to an oxidation state of 0 in Cu (s). The oxidizing agent is oxygen and the reducing agent is glucose. Oxygen is reduced, so it is an oxidizing agent. The glucose is oxidized, so it is a reducing agent.

26 Simbol Bahan Kimia Beserta Arti dan Contohnya Materi Kimia SMA
Abstract. Summary: Anaerobic ammonium-oxidizing (anammox) bacteria defy many microbiological concepts and share numerous properties with both eukaryotes and archaea. Among their most intriguing characteristics are their compartmentalized cell plan and archaeon-like cell wall. Here we review our current knowledge about anammox cell biology.

How To Find The Oxidizing and Reducing Agent YouTube
Apa itu Agen Pengoksidasi? nitiwa / Getty Images. Diperbarui pada 04 April 2020. Agen pengoksidasi adalah reaktan yang menghilangkan elektron dari reaktan lain selama reaksi redoks. Agen pengoksidasi biasanya mengambil elektron ini untuk dirinya sendiri, sehingga mendapatkan elektron dan direduksi. Agen pengoksidasi dengan demikian merupakan.

Common Oxidizing Agents & Reducing Agents ChemTalk
Cl 2 gains one electron; it is reduced from Cl 2 to 2 Cl -; thus, Cl 2 is the oxidizing agent. Exercise 9.2.2 9.2. 2: Identify reducing and oxidizing agents. Identify the oxidizing agent and the reducing agent in the following redox reaction: MnO−4 + SO2−3 → Mn2+ + SO2−4 M n O 4 − + S O 3 2 − → M n 2 + + S O 4 2 −.

PPT Chapter 19 Oxidation Reduction Reactions PowerPoint Presentation ID824481
Bahan kimia oksidator memiliki contoh yang dapat dikatakan banyak, walaupun tidak terdapat secara bebas di alam, yang pada umumnya dalam bentuk garam. Sehingga apabila ingin mendapatkan bahan kimia ini secara murni maka harus melewati beberapa proses yang dimulai dari proses elektrolisis atau melalui cara mengoksidasi senyawa halida (X -).
/GettyImages-12115858631-7ecb07de60214f9cba1e6aad1b92eb41.jpg)
Oxidizing Agent Definition and Examples
The substances oxidizers They are oxidizing substances that under specific conditions of temperature and pressure can react with a fuel and produce combustion. In this process, the oxidizer oxidizes the fuel and the fuel reduces the oxidizer. For instance: ozone, halogens, nitrates. Oxidizers are oxidizing agents, prone to highly exothermic reduction-oxidation reactions (they produce heat), so.

Warning Oxidizing Substance Symbol ,Vector Illustration, Isolate on White Background Label
An oxidizer, also known as an oxidant or oxidizing agent, is a reactant that removes electrons from other reactants during a redox reaction.It may also be considered to be a chemical species that transfers electronegative atoms to a substrate. The word origin derives from the transfer of oxygen, but the definition has since been expanded to include other species in a redox reaction.

Reaction of Oxidizing Agents, Chemistry Lecture Sabaq.pk YouTube
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent (called the reductant, reducer, or electron donor ). In other words, an oxidizer is any substance that oxidizes another substance.

Oxidizing Agent Easy Science Oxidizing agent, Science chemistry, Easy science
Oxidized - A substance is oxidized when it undergoes the process of oxidation. I.e the addition of oxygen or any electronegative elements or the removal of hydrogen or any electropositive element. To learn more about the Oxidized process, Oxidizing agents and FAQs, Visit BYJU'S.

Class 5 Oxidizing Substances and Organic Peroxides AIDGC
Oxidizing Agent Definition. An oxidizing agent is a chemical substance which causes another chemical species to lose electrons. Oxidation means the loss of electrons, the loss of a hydrogen atom, or the addition of an oxygen atom. The oxidizing agent has the ability to accept or transfer those electrons.

PPT Safety In the Microbiology Lab PowerPoint Presentation, free download ID2335239
Robby Binur 6. Yulianis Zella Alfinda ffKARAKTERISTIK Nama : Oxidizing Lambang : O Arti : Bahan kimia bersifat pengoksidasi, dapat menyebabkan kebakaran dengan menghasilkan panas saat kontak dengan bahan organik dan bahan pereduksi. fCONTOH BAHAN - BAHAN OKSIDATOR Kalium klorat (KClO₃) Kalium Permanganat (KMnO₄) Hidrogen Peroksida.

Contoh Bahan Kimia Oxidizing 11 Docx 1 Oxidizing Pengoksidasi Oxidizing Atau Bahan Kimia
The reducing agent is a substance that causes reduction by losing electrons. The simplest way to think of this is that the oxidizing agent is the substance that is reduced, while the reducing agent is the substance that is oxidized. The example below shows how to analyze a redox reaction. Example 22.3. 1. When chlorine gas is bubbled into a.
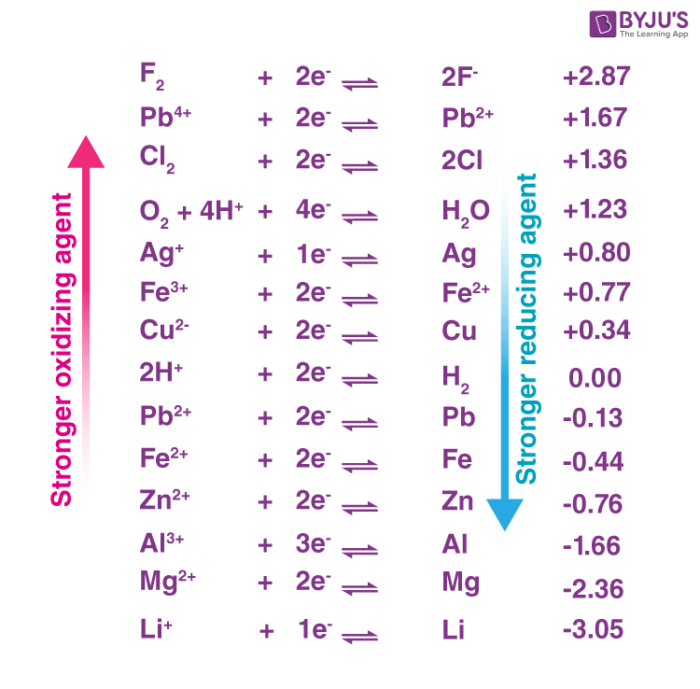
Oxidizing Agent Definition, Properties, Examples, Applications
Personnel handling oxidizing chemicals must wear adequate eye protection. Adequate safety glasses must meet the requirements of the Practice for Occupational and Educational Eye and Face Protection (ANSI Z.87. 1 1989) and must be equipped with side shields. Ordinary prescription glasses do not provide adequate protection unless they meet this.

Oxidizing and Reducing Agents — Definition & Examples Expii
Flammable (Mudah Terbakar) Bahan Kimia Mudah Terbakar. Simbol flammable hampir sama artinya dengan simbol oxidizing, bahan kimia dengan simbol flammable memiliki titik nyala api yang sangat rendah sehingga sangat mudah terbakar pada suhu panas ataupun dengan sumber api yang sangat kecil. Sama seperti pada zat pengoksidasi, bahan kimia yang.