
1.Oblicz stopnie utlenienia pierwiastków w następujących związkach SiO2, MgO, H2S, NH3, H3PO4
K2Cr2O7+H2SO4+SO2=K2SO4+Cr2(SO4)3+H2O balance the redox reaction by ion electron method or half reaction method. k2cr2o7+h2so4+so2=k2so4+cr2(so4)3+h2o.

Action Of Acidified K2Cr2O7 On Hydrogen Sulphide D and F Block Elements Chemistry Class 12
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. K2Cr2O7 + 4 H2So4 + 3 H2S = K2So4 + Cr2(So4)3 + 7 H2O + 3 S. Reactants.
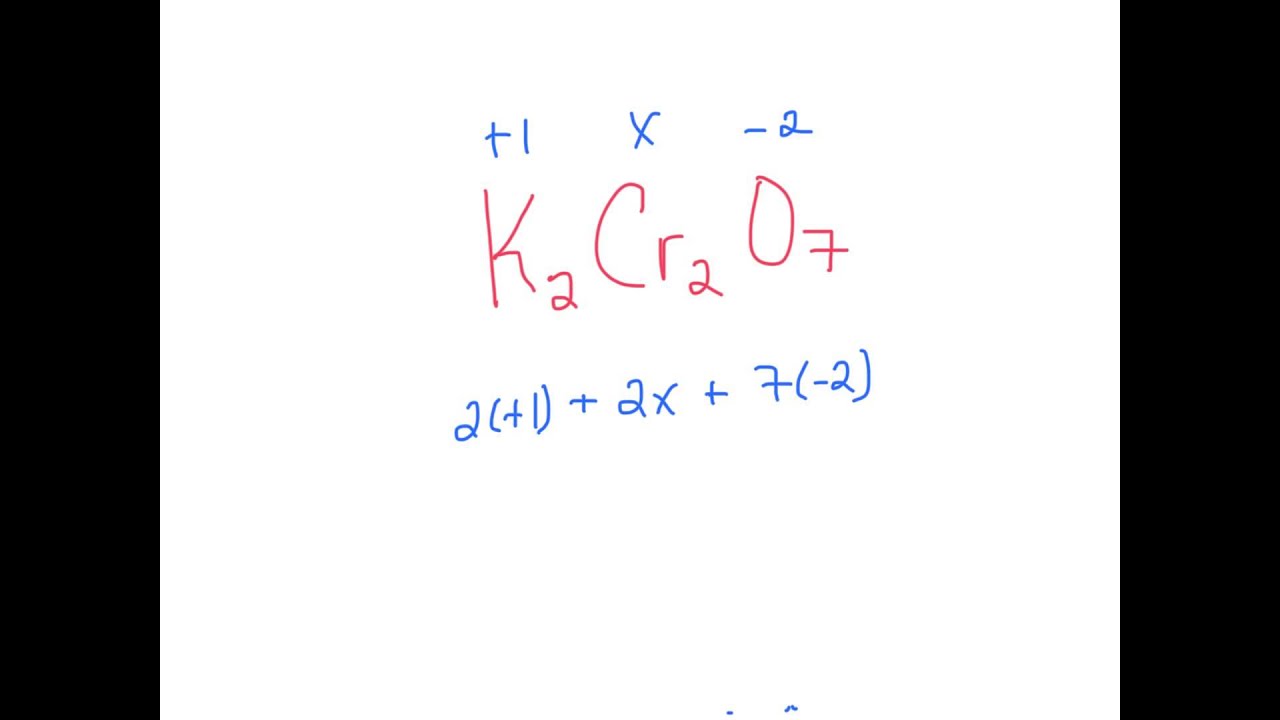
K2Cr2O7 YouTube
Here's what I got. Start by writing the unbalanced chemical equation. Potassium dichromate, "K"_2"Cr"_2"O"_7, will react with hydrosulfuric acid, which is aqueous.

K2Cr2O7 + H2S + H2SO4 =S +K2SO4 + Cr2(SO4)3 + H2O is a very common reaction. The chemistry
Step 1. Write the skeleton equation. The molecular equation is. K2Cr2O7 + H2SO4 +SO2 → K2SO4 + Cr2(SO4)3 +H2O. Strip the equation of all common ions, also H+,OH- and H2O (these come back in during the balancing procedure). K+ + Cr2O2- 7 + H+ + SO2- 4 + SO2 → K+ + SO2- 4 +Cr3+ + SO2- 4 + H2O. The above equation simplifies to. Cr2O2- 7 +SO2.

Найдите сумму коэффициентов в правой части уравнения. H2S + H2SO4 + K2Cr2O7 = Cr2(SO4)3 + S
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 0 K2Cr2O7 + 3 H2S + H2SO4 = 0 Cr2(SO4)3 + 0 K2SO4 + 4 S + 4 H2O. Reactants. Products.

Реакции выражаются схемами. H2S + Cl2 + H2O → H2SO4 + HCl, K2Cr2O7 + H2S + H2SO4 → S + Cr2(SO4)3
Balance Chemical Equation - Online Balancer. Word equation; 4 Potassium dichromate + 3 Sulfane + 13 Sulfuric acid = 4 Potassium sulfate + 4 Chromium(III) sulfate + 16 Water

K2Cr2O7 + H2So4 / Which compound can be most easily oxidized... Clutch Prep It must have
Balance the chemical equation k2cr2o7+h2so4+so2=k2so4+cr2(so4)3+H2O Balance the chemical reaction K2Cr2O7+H2SO4+SO2=K2SO4+Cr2(SO4)3+H2O Potassium dicro.

K2cr2o7 K2so3 H2so4 Cr2 So4 3 K2so4 H2o Margaret Wiegel
This reaction can be represented by the following equation: K2Cr2O7 + H2SO4 → H2CrO4 + K2SO4. Chromic acid is a strong oxidizing agent and plays a crucial role in the subsequent steps of the reaction. Oxidation of Sulfuric Acid: In the presence of chromic acid, sulfuric acid gets oxidized to form sulfur trioxide (SO3).

Презентация по Химии "Хром" скачать смотреть бесплатно
Detailed information about the equation. Reaction conditions when applied H2SO4 + K2Cr2O7 + K2S. Reaction process H2SO4 + K2Cr2O7 + K2S. The result of the reaction H2SO4 + K2Cr2O7 + K2S.

how to balance h2o4 + h3s + 1 + h2o
Detailed information about the equation. Reaction conditions when applied H2S + H2SO4 + K2Cr2O7. Reaction process H2S + H2SO4 + K2Cr2O7. The result of the reaction H2S + H2SO4 + K2Cr2O7.

Uzgodnij współczynniki w następujących reakcjach Cr2O3 + KNO3 + KOH K2CrO4 + KNO2 + H2O
The hydrogen atoms are already balanced, so we don't need to make any changes. The equation now looks like this: K2Cr2O7 + H2SO4 ---> K2SO4 + Cr2(SO4)3 + H2O + 2O2 Answer 3. Finally, we need to balance the number of potassium and sulfate ions. On the left side, we have 2 potassium ions from K2Cr2O7 and 1 sulfate ion from H2SO4.

Napisz reakcje redoks, wskaż reduktor, utleniacz i zapisz reakcje połówkowe K2Cr2O7 + H2S
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 4 K2Cr2O7 + -3 H2S + 15 H2SO4 = 4 Cr2(SO4)3 + -8 S + 8 KHSO4 + 8 H2O. Reactants. Products.

Metode Ion Elektron K2Cr2O7 + H2S + H2SO4 YouTube
This video is the practical demonstration of the reaction of Acidified Potassium dichromate (k2Cr2O7+H2SO4) with Hydrogen peroxide (H2O2).Precipitation and d.

please try to balance this equation K2Cr2O7 + H2SO4 > K2SO4 + Cr2(SO4)3 + H2O + O2 Brainly.in
H2SO4 + K2CrO4 → H2O + K2Cr2O7 + K2SO4 | Equation Balance. Color some common substances.

Используя метод инноэлектронного баланса,расставьте коэф. в окислитвосст. реакциях.Определите
Solved and balanced chemical equation K2Cr2O7 + 3 H2O2 + 4 H2SO4 → Cr2(SO4)3 + 7 H2O + K2SO4 + 3 O2 with completed products. Application for completing products and balancing equations. Chemical Equations online! Submit. Advanced search. K 2 Cr 2 O 7 + 3 H 2 O 2 + 4 H 2 S O 4 → Cr 2 (S O 4) 3 + 7 H 2 O + K 2 S O 4 + 3 O 2. This.

Ki reacts with h2so4 producing i2 and h2s the volume of 0.2 N h2so4 r askIITians
Solved and balanced chemical equation K2Cr2O7 + 3 H2S + 4 H2SO4 → K2SO4 + Cr2(SO4)3 + 3 S + 7 H2O with completed products. Application for completing products and balancing equations.. [Note: Sense of smell becomes rapidly fatigued & can NOT be relied upon to warn of the continuous presence of H2S. Shipped as a liquefied compressed gas.]