
(PDF) Manual of Medicinal and Aromatic Plants
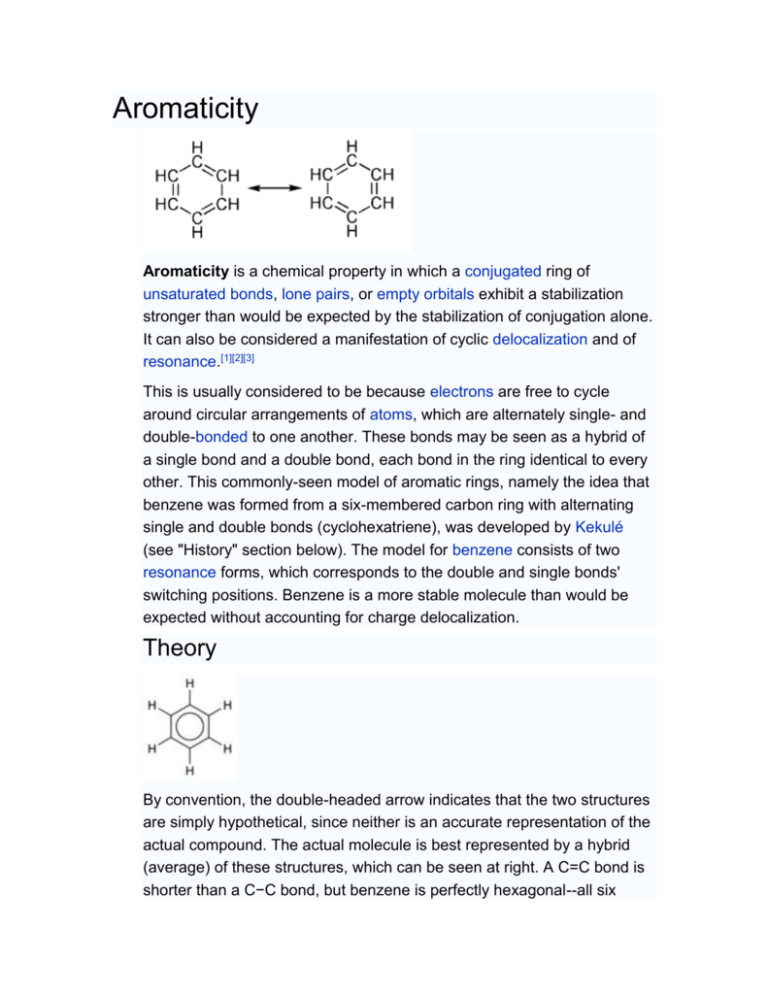
Abstract. Aromaticity/aromatic belongs to one of the most useful and popular terms in organic chemistry and related fields. However, aromaticity is not an unambiguous term; therefore, its.

Aromaticity 1st Edition
Aromaticity and Antiaromaticity A comprehensive review of the science of aromaticity, as well as its evolution, from benzene to atomic clusters In Aromaticity and Antiaromaticity: Concepts and Applications, a team of accomplished chemists delivers a comprehensive exploration of the evolution and critical aspects of aromaticity. The book examines the new global criteria used to evaluate.

Buy FUNDAMENTALS OF AROMATICITYA SIMPLIFIED APPROACH TO UNDERSTAND
20364 Altmetric 13 Citations 653 LEARN ABOUT THESE METRICS Export RIS PDF (30 KB) Get e-Alerts SUBJECTS: Aromatic compounds, Aromaticity, Electrical energy, Energy, Hydrocarbons Michael Faraday discovered benzene in 1825 ( Phil. Trans. R. Soc. London, 1825, 440).

Answer Aromaticity 09 PDF PDF
aromaticity.pdf Description: This resource includes the following topics: Introduction and Nomenclature, Stabilityof Aromatic Compounds/Huckel?s Rule, Aromatic Ions, Aromatic Heterocycles, and Polycyclic Aromatic Compounds. Resource Type: Lecture Notes pdf 678 kB aromaticity.pdf Download File DOWNLOAD

Aromaticity PDF
Aromaticity and Antiaromaticity | Wiley Online Books Aromaticity and Antiaromaticity: Concepts and Applications Author (s): Miquel Solà, Alexander I. Boldyrev, Michał K. Cyrański, Tadeusz M. Krygowski, Gabriel Merino First published: 14 October 2022 Print ISBN: 9781119085898 | Online ISBN: 9781119085928 | DOI: 10.1002/9781119085928
(PDF) Aromaticity, Antiaromaticity, Homoaromaticity and the Hückel (4n
Constructing Molecular Orbitals • molecular orbitals are the sideways overlap of porbitals •porbitals have 2 lobes.Plus (+) and minus (-) indicate the opposite phases of the wave function, not electrical charge •When lobes overlap constructively, (+ and +, or - and -) a bonding MO is formed

Aromaticity in benzenoid and non benzenoid compounds pdf systemlasopa
Aromatic Dianions I 1H NMR: d6.75, singlet Cyclooctatetraene anti-aromatic tub-shaped Cyclooctatetraenyl anion aromatic planar 1H NMR: d5.56, singlet 2 Na = Aromatic Dianions II Dihydropentalene H 2 n-butyllithium H H H Pentalene dianion Pentalene. The End. Created Date:
Full article Overview of Polycyclic Aromatic Compounds (PAC)
Aromaticity. Page ID. William Reusch. Michigan State University. The adjective "aromatic" is used by organic chemists in a rather different way than it is normally applied. It has its origin in the observation that certain natural substances, such as cinnamon bark, wintergreen leaves, vanilla beans and anise seeds, contained fragrant compounds.

Reference Book Maria Mitchell Herbalist
Aromaticity and Antiaromaticity A comprehensive review of the science of aromaticity, as well as its evolution, from benzene to atomic clusters In Aromaticity and Antiaromaticity: Concepts and Applications, a team of accomplished chemists delivers a comprehensive exploration of the evolution and critical aspects of aromaticity.

aromatic1.pdf Aromaticity Ion
Aromaticity is one of the most deeply rooted concepts in chemistry. But why, if two-thirds of existing compounds can be classified as aromatic, is there no consensus on what aromaticity is? σ−, π−, δ−, spherical, Möbius, or all-metal aromaticity… why are so many attributes needed to specify a property? Is ar 2023 Chemical Science Perspective & Review Collection Emerging.
Rules for Aromaticity The 4 Key Factors Master Organic Chemistry
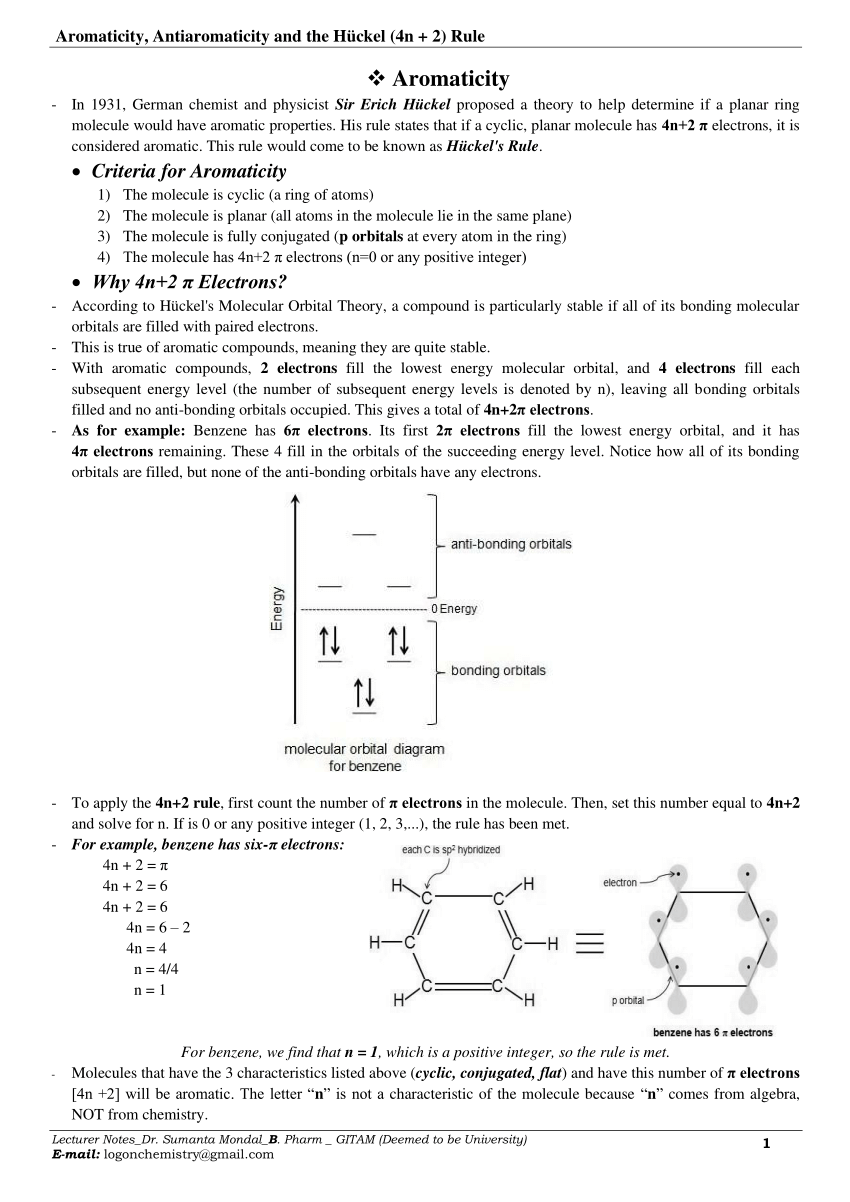
According to Hückel's Molecular Orbital Theory, a compound is particularly stable if all of its bonding molecular orbitals are filled with paired electrons.This is true of aromatic compounds, meaning they are quite stable. With aromatic compounds, 2 electrons fill the lowest energy molecular orbital, and 4 electrons fill each subsequent energy level (the number of subsequent energy levels is.
Aromaticity
the aromaticity of the molecule) and a magnetic criterion (existence of the diamagnetic ring current induced in a conjugated cyclic molecule by an external magnetic. This is a PDF rendering of the IUPAC Gold Book term 'aromaticity' Created Date: 2/24/2014 12:30:48 PM.

(PDF) Hand Book of Medicinal and Aromatic Crops
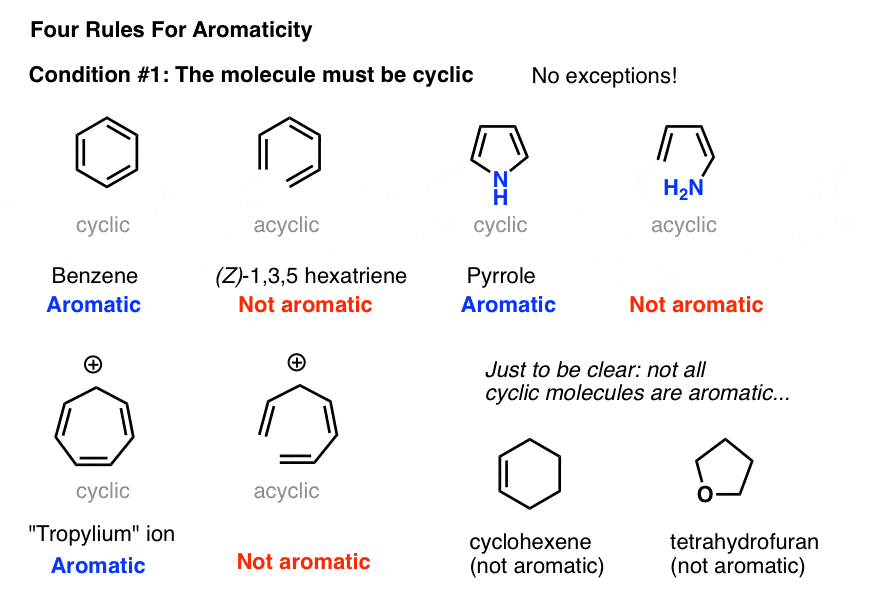
This rule would come to be known as Hückel's Rule. Criteria for Aromaticity 1) The molecule is cyclic (a ring of atoms) 2) The molecule is planar (all atoms in the molecule lie in the same plane).

Aromatic Chemistry Textbook
TrimSize:8inx10in Klein5e-Vol2 c01.tex V1-September13,2019 5:12P.M. Page3 1.2 NOMENCLATURE OF AROMATIC COMPOUNDS 3 CH 3 Toluene OH Phenol

Aromatic Compounds.pdf Aromaticity Pyridine
uninterrupted cyclic cloud of π electrons above and below the plain of the ring. The German. Chemist Erich Hückel was the first one to recognize that an aromatic compound must have an odd number of pairs of electrons, which can mathematically be written as 4n+2 (n = 0,1,2,3 etc).

(Download) "Aromaticity in Heterocyclic Compounds" by Tadeusz Marek
The Annulenes. Annulenes are monocyclic compounds with alternating double and single bonds. Annulenes are named using a number in brackets that indicates the ring size. Benzene is [6]annulene and cyclooctatetraene is [8]annulene. An annulene is aromatic if it has 4n+2π electrons and a planar carbon skeleton.