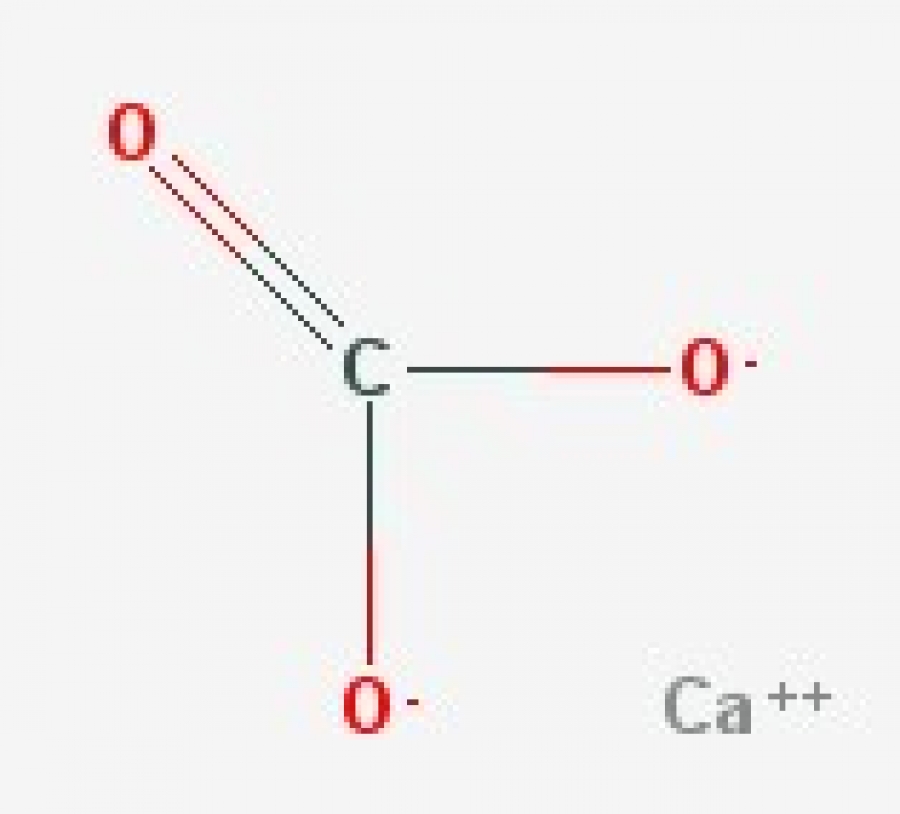
CaCO3 (Calcium Carbonate)
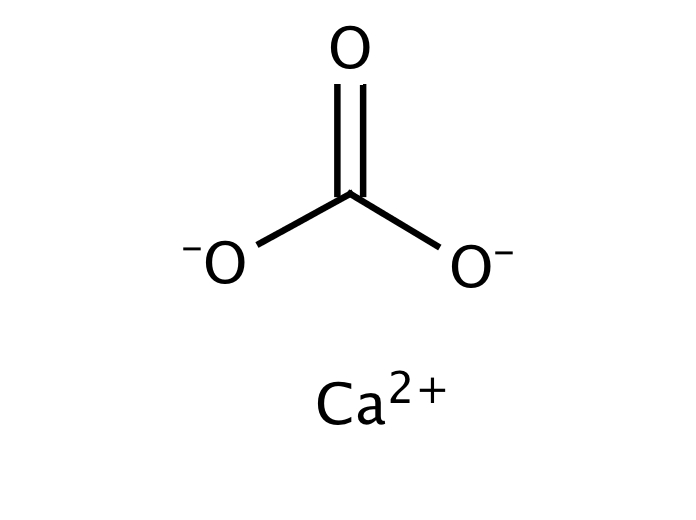
Formula and structure: The calcium carbonate chemical formula is CaCO 3 and its molar mass is 100.0869 g mol-1.The molecule is formed by the calcium cation Ca +2 and the carbonate anion CO 3-2.The structure of the calcium carbonate lattice depends on the mineral from it is extracted: the calcite contains a hexagonal calcium carbonate structure while the aragonite contains orthorhombic lattice.

How to Find the Number of Atoms in CaCO3 (Calcium carbonate) YouTube
Precipitated calcium carbonate (CAS: 471-34-1) is produced industrially by the decomposition of limestone to calcium oxide followed by subsequent recarbonization or as a by-product of the Solvay process (which is used to make sodium carbonate).
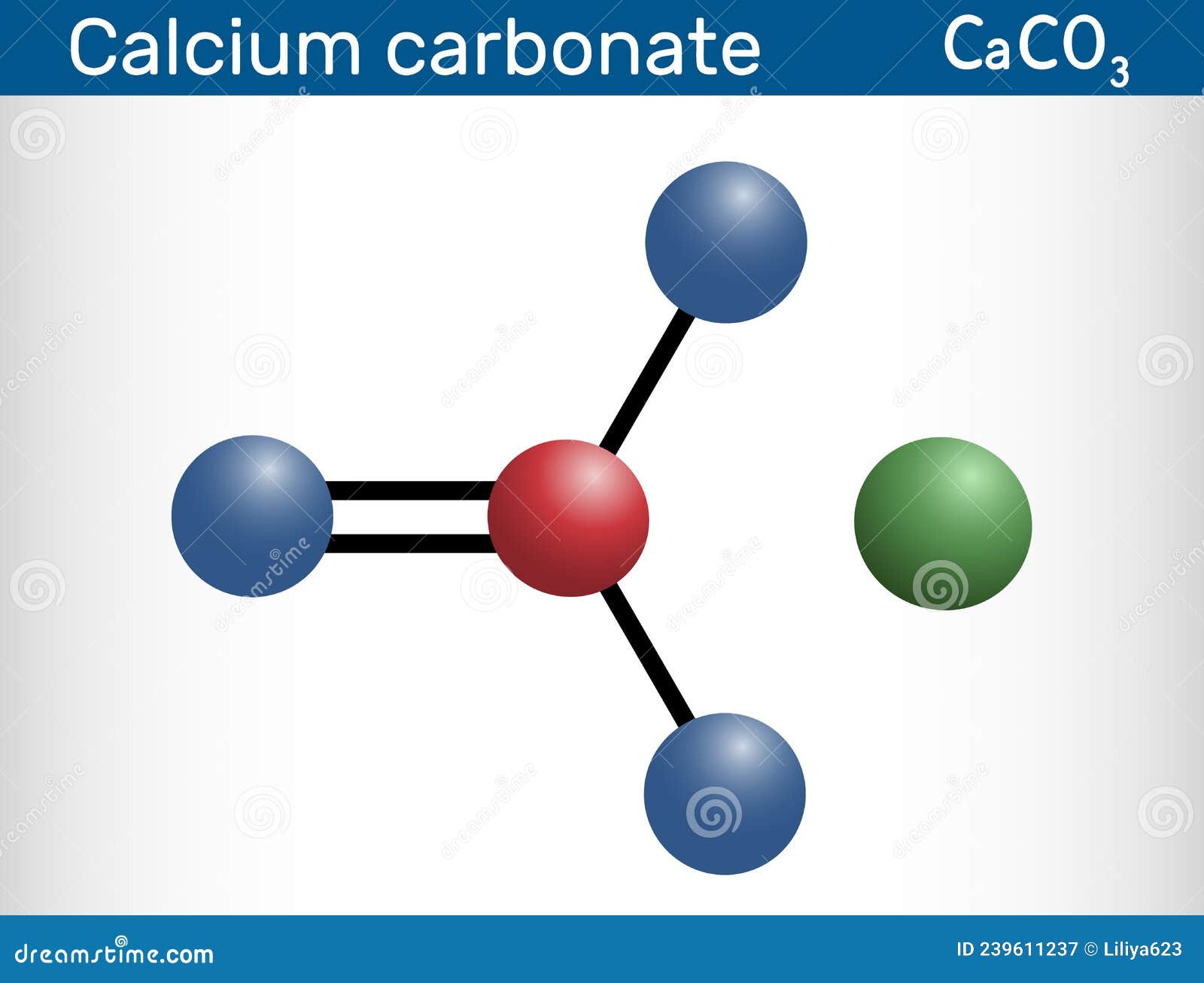
Calcium Carbonate Molecule. it is an Ionic Compound, the Carbonic Salt
Calcium carbonate Formula: CCaO 3 Molecular weight: 100.087 IUPAC Standard InChI: InChI=1S/CH2O3.Ca/c2-1 (3)4;/h (H2,2,3,4);/q;+2/p-2 IUPAC Standard InChIKey: VTYYLEPIZMXCLO-UHFFFAOYSA-L Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript .

CaCO3 Chemical & Common Name, Molar & Atomic Mass
Molecular Formula CaCO3 CCaO3 Synonyms Aragonite CALCIUM CARBONATE 471-34-1 Limestone Calcite View More. Molecular Weight 100.09 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Parent Compound CID 767 (Carbonic Acid) Component Compounds CID 767 (Carbonic Acid) CID 5460341 (Calcium) Dates Create: 2004-09-16 Modify: 2024-01-06

Calcium carbonate(CaCO3), Honeywell Fisher Scientific
Calcium carbonate is a chemical compound with the chemical formula Ca CO 3. It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous.

3 CaCO3 Physical Sciences Science
Calcium carbonate (CaCO 3) is the most commonly used filler in the plastics industry. It is not only used with several thermoplastics, such as PVC (e.g., in PVC pipes and floor tiles), but also with thermosets such as polyesters (in sheet molding compounds). Calcium carbonate is abundantly available in nature as limestone,.

Caco3 calcium carbonate 3D model TurboSquid 1423526
Appearance Calcium carbonate appears as a white, odorless powder or solid with a crystalline structure. Sample reactions for CaCO3 Related compounds Formula in Hill system is CCaO3 Computing molar mass (molar weight) To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use:
Calcium Carbonate Molecule Photograph by Laguna Design/science Photo
Molecular weight calculation: 40.078 + 12.0107 + 15.9994*3 Percent composition by element Element: Calcium Symbol: Ca Atomic Mass: 40.078 # of Atoms: 1 Mass Percent: 40.043% Element: Carbon Symbol: C Atomic Mass: 12.0107 # of Atoms: 1 Mass Percent: 12.000% Element: Oxygen Symbol: O Atomic Mass: 15.9994 # of Atoms: 3 Mass Percent: 47.957%

3D image of Calcium carbonate skeletal formula molecular chemical
Calcium carbonate, CaCO 3, is one of the most common compounds on Earth, making up about 7% of Earth ' s crust. It occurs in a wide variety of mineral forms, including limestone, marble, travertine, and chalk. Calcium carbonate also occurs combined with magnesium as the mineral dolomite, CaMg (CO 3) 2.

CaCO3 Chemical & Common Name, Molar & Atomic Mass
Calcium carbonate is an inorganic chemical compound with the chemical formula CaCO 3. Calcium carbonate is one of the most popular chemicals which is first encountered in school classrooms, where the use of chalk (a form of CaCO3) is found. It is found in the earth's crust. It is also found in many forms such as marble, limestone, etc.
Fragments of the crystal structures of (a) CaCO3 (calcite), (b
calcium carbonate (CaCO3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. Calcium carbonate is either a white powder or a colorless crystal.
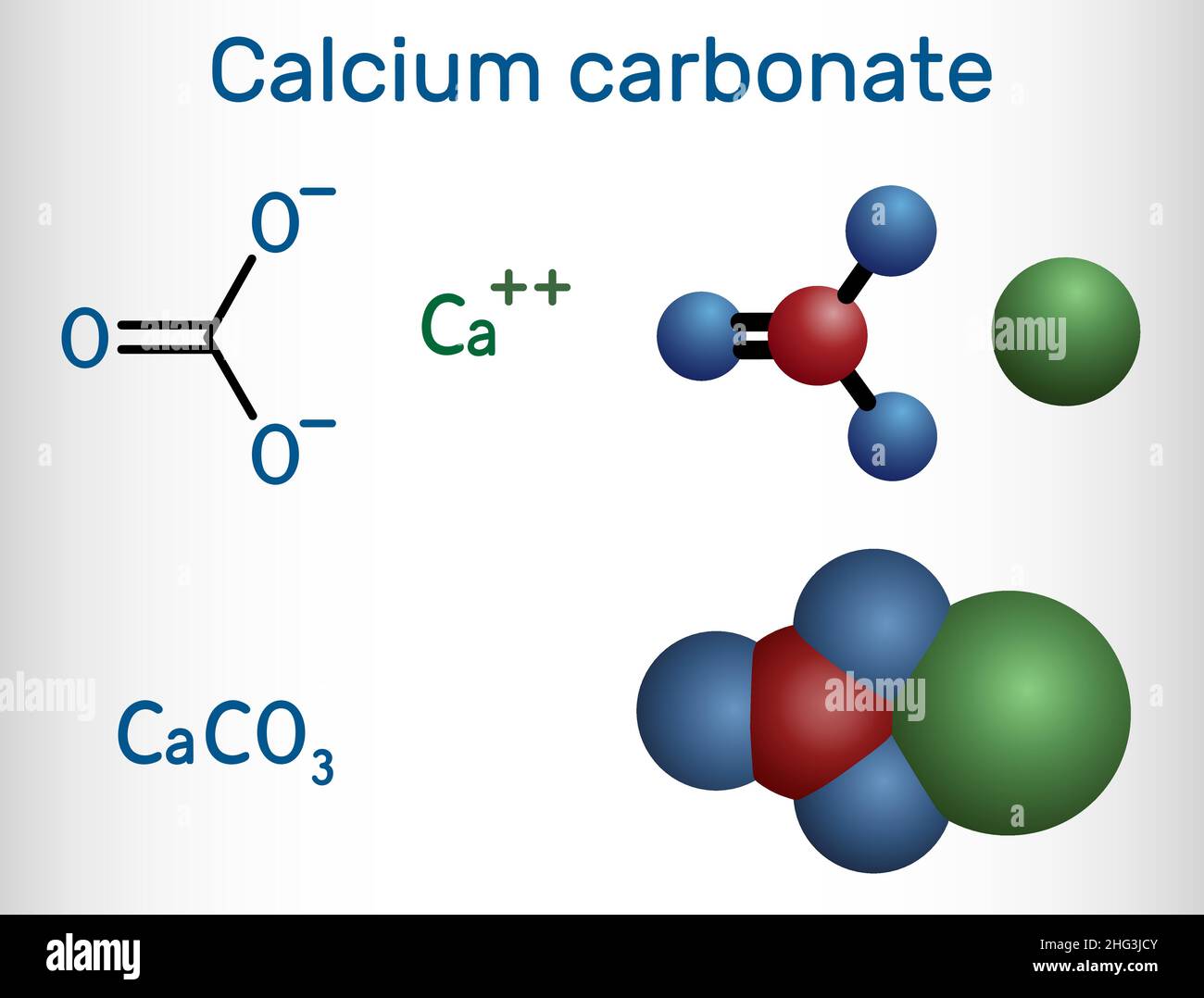
Calcium carbonate molecule. It is an ionic compound, the carbonic salt
Calcium Carbonate Formula The chemical formula of calcium carbonate is CaCO₃. This means that each molecule of calcium carbonate contains one calcium atom, one carbon atom, and three oxygen atoms. The formula is important in determining the amount of calcium carbonate needed for a specific application and in understanding its chemical properties.

Calcium Carbonate 3D Model CaCO3 free 3D model CGTrader
simple solution method 1. Introduction Calcium carbonate (CaCO3) is an abundant inorganic biomaterial in the manner of different structures (calcite, vaterite, and aragonite) [1, 2 ]. Thermodynamically, the form of CaCO 3 under normal conditions is β-CaCO 3 (calcite).
Purchase Calcium carbonate [471341] online • Catalog • Molekula Group
Carbonates. Carbonate is a polyatomic anion with the formula CO2−3 C O 3 2 − and has a trigonal planar molecular structure which consists of a carbon atom surrounded by three oxygen atoms. The carbonate ion is a moderately strong base, so by definition of a Lewis base, it attracts protons in aqueous solutions.
Molecular structure calcium carbonate caco3 Vector Image
Calcium Carbonate is a chemical compound having the chemical formula CaCO3. It is a white and insoluble powder-like substance that occurs naturally in minerals, marble, chalk, limestone, shells, calcite, pearl, and other related compounds. Medicinally, we use it as an antacid or a calcium supplement. It can also be used as cosmetics fillers.
Caco3 molecule fotografías e imágenes de alta resolución Alamy
Calcium carbonate is a chemical compound with the formula CaCO3 formed by three main elements: carbon, oxygen, and calcium. It is a common substance found in rocks in all parts of the world (most notably as limestone), and is the main component of shells of marine organisms, snails, coal balls, pearls, and eggshells.